Prof. Cibulka: Scandium(III) previously considered redox-inert is a surprising catalyst in reactions driven by visible light
The results of Professor Cibulka's group on the novel use of scandium compounds in organic synthesis were published in the prestigious journal Nature Communications.
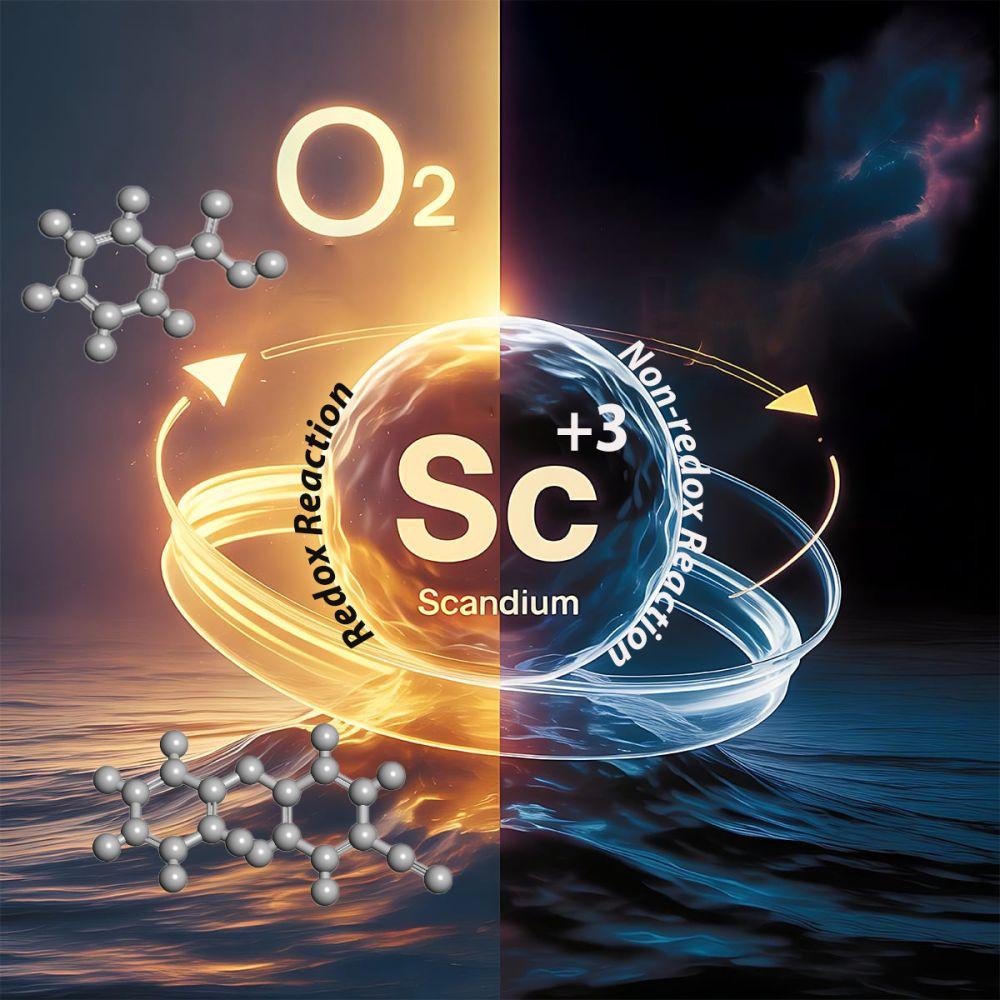
The study demonstrates that scandium salts – specifically scandium triflate – can serve as photocatalysts. Upon irradiation with visible light, they enable aerobic oxidations and other transformations of compounds containing an aromatic core. This discovery is unexpected, as scandium ions are generally considered redox-inert, i.e., not undergoing changes of oxidation state.
The results were obtained within the Eco&Stor project coordinated by the University of Chemistry and Technology, Prague, supported by the Excellence in Research program. A team of scientists from the Institute of Chemical Technology, Prague worked on the results published in the article in cooperation with foreign colleagues from Saudi Arabia and Egypt and with colleagues from the J. Heyrovský Institute of Physical Chemistry of the Czech Academy of Sciences.
"The discovery that scandium compounds can be excited with visible light and used to catalyse oxidation reactions was completely unexpected. We therefore spent many months verifying our observations with different methods to ensure the results were solid," says team leader Professor Cibulka, adding: "The finding points to a completely new application of scandium – a metal so far rather neglected in catalysis, even though it is relatively abundant on Earth."
Publication link: https://doi.org/10.1038/s41467-025-63233-4







