Fighting climate change: designing hierarchically porous materials for carbon dioxide (CO2) capture
Atmospheric CO2 concentrations continue to increase as a result of human activities and are thus considered to be either the reason for or the result of climate change. Although the implications of CO2 in climate change entered the public dialogue several decades ago, significant confusion surrounding the topic remains. Many people are unmoved by the issue or think of it as too complex a problem to even think of fixing. Many governments and stakeholders claim that climate change is a worldwide hoax invented by scientists to fool the general public, despite all the obvious consequences already occurring: unpredictable weather patterns, melting of the Arctic sea ice, floods, heat waves, shifting rainfall patterns, and rising sea levels. But the global scientific community has proven that anthropogenic carbon emissions from human activities are the main contributors to the greenhouse effect, trapping heat and making the earth warmer[1].
We can choose to take action now and slow the rate of climate change, or we can alternatively pretend that all of this is not true and hope for the best, undermining the science behind the issue. Herein, the preparation of low cost adsorbents for CO2 capture is presented.
Considering the fact that the burning of fossil fuels is the major culprit to the rising atmospheric CO2 concentrations, the most logical solution for combatting climate change will be to switch to renewable (clean) energy sources such as solar or wind systems. However, the main hurdle still holding us back is the fact that coal and oil are abundant and cheap, while current renewable energy sources are not yet able to meet increasing global energy demands. Therefore, while we develop and search for new alternative clean energy sources, we can still be more efficient in our energy use, plant more trees, and reduce greenhouse gas emissions, especially from large emission sources such as fossil fuel burning power plants and by applying carbon capture and storage (CCS) technologies. Since there has been a delay in cutting CO2 emissions, considering how long it has been since global warming and climate change came into the spotlight, we need multiple solutions simultaneously. In addition to reducing industrial emissions, we need to start removing much of the CO2 which has already been released into the atmosphere. An increase in global temperatures by 2° C is considered the point of no return, which means we need urgent action. Many nations are therefore committed to limiting their greenhouse gas emissions in order to keep global temperature increase below 1.5° C. The development of new high performance materials and technologies for capturing CO2 in economical and environmentally friendly ways is key to achieving this target. Because CO2 capture is the most expensive part of CCS technology, most research has focused on reducing the costs of sorbents. So far, solid sorbents are the most promising due to their durability over several cycles and their low energy requirements for regeneration.
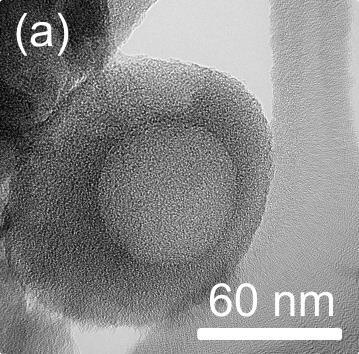
In the Bioengineering and Functional Materials Laboratory, a large part of our research activities centers around the design of multifunctional nanostructured materials for various applications (CO2 capture, bioseparations, biomedicine etc.) in parallel to mathematical modelling of underlying mechanisms e.g. nanoparticle aggregation. Our most recent work[2] highlights the development of hierarchically porous materials for CO2 capture using an easy and new concept from cheap precursors (polystyrene and polyaniline), without the need for any complicated instrumentation. Our strategy (summarized in Scheme 1) is based on the removal of sacrificial polystyrene nanoparticles (PS NPs) during a pre-carbonization step which generates large pores for easy gas diffusion followed by chemical activation to generate micropores, which are required for high capacity CO2 capture. Transmission and scanning electron microscopies have revealed the hierarchically porous nature of the materials (Figure 1
and 2 respectively). By varying the size of the sacrificial PS NPs and the intensity of chemical treatment, the material pore size distribution was easily tuned. We obtained an outstanding CO2 capture capacity with our optimum material (9.14 mmol g-1 at 273.15 K and 1 bar), which is one of the highest sorption capacities reported for carbon materials in the literature. Interestingly, a linear relationship between CO2 sorption capacity and the ultramicropore volume (pores < 0.7 nm) was observed for all the prepared materials (Figure 1 [c]), thus highlighting the importance of very small pores in the capture performance. Given the performance of these materials and the simplicity in their preparation, we believe they are promising CO2 sorbents, and the approach could serve as a guide to other researchers who might be interested in the design of new high-capacity CO2 capture sorbents.
To be commercially attractive, in addition to high sorption performance, an adsorbent should also be capable of being easily regenerated and mechanically strong enough to withstand the bulk handling typical of industrial units. As such, our future work will focus on evaluating the stability of these sorbents under various industrial conditions as well as the ability to be regenerated after multiple adsorption/desorption cycles.
If we fail to keep CO2 emissions under control, the effects might be irreversible, and it will be too late to fix, rendering our home—the Earth—unlivable. There is still much to be done and a long path ahead of us; however, we believe that carbon capture and storage systems, if developed, could contribute significantly to mitigating the climate change problem.
References
- IPCC Fifth Assessment, 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, F., D. Qin, G.-K. Plattner, Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1535 pp.
- Edith Mawunya Kutorglo, Fatima Hassouna, Anna Beltzung, Dušan Kopecký, Ivona Sedlářova and Miroslav Šoóš, (2018) https://doi.org/10.1016/j.cej.2018.10.133.
Author is a PhD student at Department of Chemical Engineering







